July 9, 2020 19:36 by
 admin
admine-ETP TREAT EFFLUENT USING ADAVNCED OXIDATION PROCESSES (AOP)
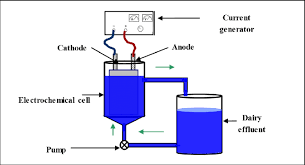
Conventional oxidation treatment have found difficulty to oxidize dyestuffs and complex structure of organic compounds: At low concentration or If they are especially refractory to the oxidants. The AOP as Electro Oxidation processes are combination of : Ozone (O3), - Hydrogen peroxide (H2O2) , Hypo chlorous (HOCl) Electro Chlorination(NaOCl) and - which showed the greatest promise . These oxidants effectively decolorized dyes, however did not remove COD completely. The goal of any AOPs design is to generate and use hydroxyl free radical (HO·) as strong oxidant to destroy compound that can not be oxidized by conventional oxidant.
OZONATION : Ozone is a powerful oxidant agent for water and wastewater. Once dissolved in water, ozone reacts with a great number of organic compounds in two different ways: By direct oxidation as molecular ozone or By indirect reaction through formation of secondary oxidants like hydroxyl radical. The conventional fine bubble contactor is the most widely ozone generator used because of the high ozone transfer efficiency (90%) and high performance. Results presented by a few researchers revealed that ozone decolorize all dyes, except non soluble disperse and vat dyes which react slowly and take longer time. Colour removal using ozonisation from textile wastewater is depended on dye concentration. The attached image is self explanatory.